Adding Value Chain
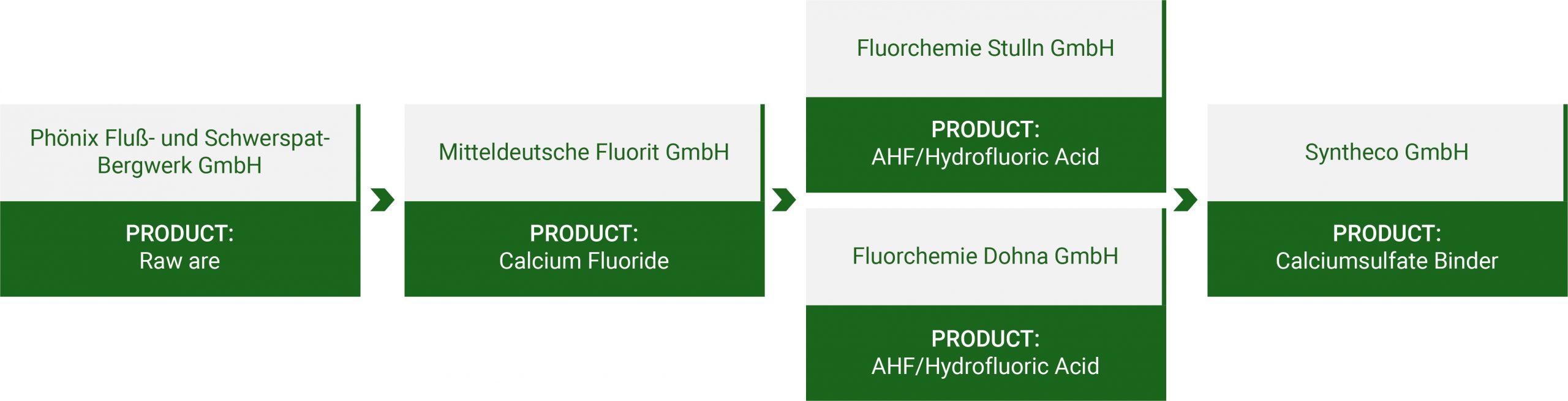

Hydrofluoric Acid (HF) is an important chemical in many industrial markets. HF is a useful provider of elemental fluorine as its unique properties play an important role in the manufacture of many technical products in daily use.
The applications of fluorine in this form are numerous. They range from additives for cleaning aluminium wheels, decontamination of stainless steel to weather resistant plastic fibre for outdoor clothing, to glass treatment as well as additives in tooth paste.
Calcium sulphate binder is mainly used in the production of screeds and flowing screeds. Flowing screeds with a calcium sulphate base are becoming increasingly popular due to their easy application, self-leveling properties and quick setting.
In comparison to cement, screeds with a calcium sulphate base demonstrate a considerably lesser tendency towards degradation. In addition, the typical propensity of cement to disintegrate around the corners is entirely absent.
Calcium sulphate screeds also demonstrate low thermal expansion, which makes them an ideal material for use with in-floor heating.
Further usages for calcium sulphate screeds are in putty and plaster.
Hexafluorosilicic acid, or Fluosilic Acid for short, is mainly used in the production of cryolite and aluminium fluoride. Both substances are used in the aluminium industry.
Fluorides are produced when the chemical element fluorine binds with other chemical elements. Fluorine is found mainly in the mineral fluorite (also known as fluorspar). It is a naturally occurring mineral which can contain up to 45% calcium fluoride (CaF2). Fluorite is often to be found alongside other naturally occurring minerals such as barite (heavy spar), galenite (blue lead), pyrite (fools gold) and other sulphides. In its’ pure form, fluorite is colourless, see-through to translucent and has a glassy sheen. Impurities can cause the mineral to appear in a diverse range of colours, some varieties are also fluorescent. Fluorite is one of the most exquisitely colourful of the world’s natural minerals and occurs in a many different hues, from yellow or green through to pink, orange and reddish tones, right through to blue and black.
Today the largest deposits of fluorite are to be found in China, Mexico, Mongolia, South Africa and Namibia. There are still some profitable extraction sites in Europe; however their numbers have been in constant decline over the years. The mining of fluorite is an international enterprise and the worldwide consumption of the mineral is estimated to be around 4.5 million tonnes per year.
The principle grades of fluorite are:
With a worldwide production capacity of over 3 million tonnes, acid grade fluorite, used as the base material for hydrofluoric acid, is most in demand.
Following the excavation of the ore through mining or quarrying, the impurities are removed leaving the fluorite with a calcium fluoride content of no less than 97%. The majority of the by-products are recycled and used in a variety of industrial purposes. The acid grade fluorite is taken by road, rail or ship to the hydrofluoric acid production sites. Here it is decomposed with sulphuric acid to produce hydrogen fluoride gas. This is either stored as a liquefied gas, or mixed with water to produce an aqueous hydrofluoric acid solution until use.